



 |



I got involved in this battle for rationality
because of the most
basic physics I learned as a grade school nerd in the 1950s .
In the mid '70s , I learned Ken Iverson's APL to
ease my learning the multidimensional geometry I needed to grok papers which seemed to be saying something
non-trivial .
Since then , for me , to understand something is to be
able to compute it . So , I approach the understanding of
mean
planetary temperature from a very classical physical reductionist
perspective looking at how much of the unexplained delta between our
known physics and our observations is reduced by by each added
parameter .
Joe Bast invited me to start a page on their ClimateWiki taking
this essential
physics approach . So far , on that page , I work thru the
calculation of the temperature of a uniform gray ball in our orbit in
an evolute of Iverson's array programming language , Arthur Whitney's K
. Tables are set up for others to translate the quantitative
relationships into other programming notations . I use Arthur's K
here but the expressions are generally quite similar to their
traditional textbook equivalents . It's just that they operate on
entire lists .
Tball ~ Tgray * ( a % e ) ^ % 4 / where ~ is the function "identically equal"where Tgray is the StefanBoltzmann calculated temperature for a flat spectrum , ie , gray body . The temperature of a uniformly colored , ie , non-flat spectrum body is the gray body temperature times the fourth root of the ratio between its absorptivity with respect to its sources and its emissivity with respect to it's sinks . For a flat-spectrum , absorptivity-emissivity is constant across all wavelengths so that ratio is necessarily 1 . So a gray ball comes to the temperature calculated by simply adding up the energy impinging on a point in our orbit as calculated on the essential physics page .
Now , how are absorptivity and emissivity for a radiantly heated uniformly colored ball computed ? At this point it becomes very hard to find any explanation on the web .
Here's the computation simply using Planck thermal radiation spectra . First , we need to calculate the spectra of source , the sun , and the equilibrium temperature of a neutral gray ball in our orbit
The Planck function , the crowning achievment of 19th century physics , provides the spectrum for a black ( totally absorptive-emissive ) body as a function of temperature . Particularly for lower temperatures , it has very long tails towards longer wavelengths . We will start by calculating the energy spectrum for the sun in our orbit , and that of a body whose temperature is such as to emit the same total energy . First we create a list of wavelengths in the range of interest . ( The definitions of the defined words are in a table below . )
WL : apv[ .1 ; .1 ; 900 ] * 1e-6 / WaveLengths ; m % cycleCreates a list of 900 wavelengths , WL , from 0.1 microns to 90 microns is steps 0.1 micron .
We will use the commonly cited temperature of 5778 kelvin for the sun's temperature , and the temperature of a gray ball in our orbit , 278.68 , calculated to match the same total energy using the StefanBoltzmann law as we'll see in a moment .
Spectra : pi * 1e-7 * Planckl[ WL ]' 5778 278.68This expression produces a 2 column table of power density for each ( the tick mark , ' , means each ) of the wavelengths in WL for each of the 2 temperatures . The 1e-7 scales the power to the 0.1 micron increment . Note that Planckl returns power per steradian while the total energy is emitted over a hemisphere . Thus the factor of pi .
We can test the accuracy of these curves and the extent to which our range of wavelengths is sufficient to include all the energy by comparing the sum across the Spectra to the total energy calculated by the Stefan Boltzmann law which , incidentally , pre-dates Planck's function .
( +/' Spectra ; T2Psb 5778 278.68 )
| 6.319533e+007 | 6.320098e+007 |
| 339.3557 | 342.0085 |
%/ r />/ 0.9999106 0.9922434 / r contains the result of the last operationThe first column is the sum across each of the computed spectra , the second , the total power calculated by StefanBoltzmann for each temperature .
Note that even going out to 90 microns , we're still off by about 0.8% on the 279k spectrum . This may not seem like much , but the commonly agreed upon total change in our temperature since before the industrial revolution is from about 288 kelvin to 288.8 , only about 0.3% . But all these numbers are not as precise as often portrayed . NoTricksZone has an interesting post showing disagreement in our mean temperature of more than 2 degrees . There are lots of ways to increase the precision of these computations , but , simple , first .
You can see above what an enormous difference in radiance that 4th power StefanBoltzmann relationship creates . In fact , the sun radiates more than 500 times as much as a 279k ball in our orbit even at the ball's peak wavelength around 10 microns .
But the disk of the sun covers only 5.41e-6 of the total celestial sphere . Scaling the sun's spectrum by that factor ,
Spectra : Spectra * 5.41e-6 1.0 / multiplies the 1st column by the 1st number , etc .We get the values plotted below :
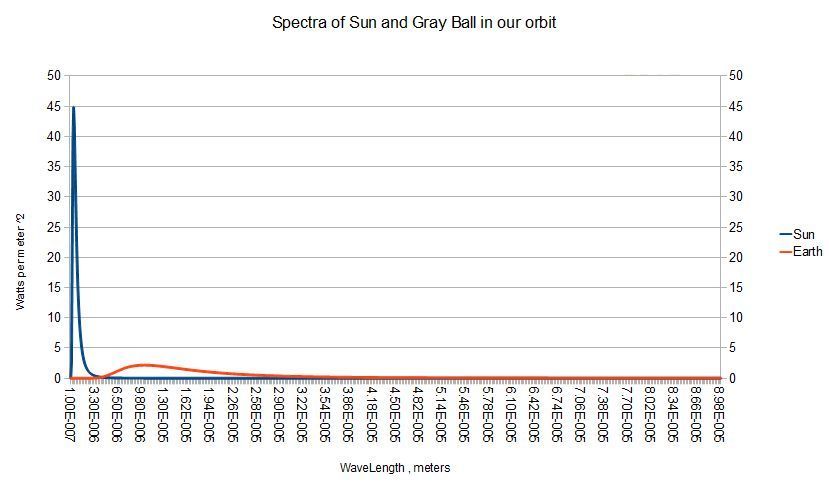
+/' Spectra />/ 341.8868 339.3557 / total radiances = areas under the curvesHere are the definintions of the words used so far :
P2Tsb r />/ 278.66 278.14 / corresponding temperatures
| apv | Arithmetic Progression Vector . ( start ; increment ; count ) / old IBM term | { x + ( ! z ) * y } |
| Planckl | W % sr * m ^ 2 from meters % cycle / Planck fn in terms of WaveLength | {[ l ; T ] ( ( 2 * h * c ^ 2 ) % l ^ 5 ) % ( _exp ( h * c ) % l * boltz * T ) - 1 } |
| T2Psb | Stefan-boltzmann : Watts % meter ^ 2 from Kelvin Temperature | {[ T ] sb * T ^ 4 } |
| P2Tsb | Stefan-boltzmann : Kelvin from Watts % meter ^ 2 | {[ P ] ( P % sb ) ^ % 4 } |
| h | Planck's constant / Joule * second | 6.626069e-034 |
| c | 'speed' of light / m % s | 2.997925e+008 |
| boltz | Boltzman constant / Joule % Kelvin | 1.380649e-023 |
Colored Balls
At any given wavelength , an opaque object may either absorb radiation of that wavelength converting it in to thermal motion or reflect it .
Over 150 years ago , Kirchhoff and others proved that since they were just two directions thru the same process , absorptivity and emissivity are always equal at each frequency . For a gray flat-spectrum body , ae = constant , across all wavelengths . Therefore the constant drops out which is why we can the temperature of the spectrum above is that of a gray body , however light or dark , not just as frequently asserted , a black body .( reflect + ae ) = 1 / at each wavelength
Note : I rather hate the term albedo which is a synonym for reflectance . Its imprecise usage causes much confusion . As we've asserted , reflectance or Albedo , is a function of wavelength . It should either be expressed as "albedo with respect to some spectrum" , or , unmodified , should refer to a flat spectrum , in which case it drops out of the equation for temperature .
As can be seen in the graph above , the solar spectrum and the thermal spectrum for a gray body in our orbit are almost disjoint . Therefore it 's acceptable to split the spectrum into short-wave ( solar ) and long-wave ( planetary ) portions . The crossover point can be found by looking for the first place the earth spectrum is greater than the solar :
That happens at index 43 , about 4.4 microns .fst1s : { x > 0 , -1 _ x } / returns indices of first 1s in strings of 1s
& fst1s @ </ Spectra />/ ,43
WL r />/ ,4.4e-6 / r contains the result of the last operation .
Going back to Hertzberg's expression , we can calculate the ratio between the mean absorptivity over the sun's spectrum to the emissivity over the planet's . Given the temperatures used above , 288 and 288.8 for the observed , and 278.68 for Tgray , we get
( 288. 288.8 % 278.68 ) ^ 4 />/ 1.140635 1.153362The commonly used value for the lumped earth+atmosphere absorptivity with respect to the sun's spectrum is 0.7 . So we can make a step function spectrum breaking at 4.4 microns , with an absorptivity of 0.7 over the short wavelengths , and
.7 % r />/ 0.6136932 0.6069215We'll check just the first of these , the 288k ratio .
AEspectrum : ,/ ( 43 , 900 - 43 ) #' 0.7 , * rThis line creates a list of 900 items , the first 43 of which are 0.7 , and the remainder 0.613... .
The line below uses K's secant search to find the temperature x whose spectrum weighted by AEspectrum matches the area of the scaled sun's spectrum weighted by the same AEspectrum .
?[ { -/ +/' AEspectrum */: ( Spectra 0 ; pi * 1e-7 * Planckl[ WL ] x ) } ; 0.0 ; 300 ]About 0.4 degrees off . ( The parameters of the search function are : ?[ function ; goal ; initialGuess ] . )
288.38
The ubiquitous null hypothesis seemingly accepted by all sides in the AGW debate is that the surface of the earth has an ae with respect to the sun of that observed 0.7 , but , that of an absolute black body , 1.0 , in the longer wavelengths . That's the most extreme assumption that can be made given the 0.7 short wave length ae . Here's what we get :
AEspectrum : ,/ ( 43 , 900 - 43 ) #' 0.7 , 1.0Here are the Sun and planet spectra and the stepped ae spectra which match our observed temperature ( green ) , the frozen planet assumption used to scare everbody ( black ) , and a flat spectrum with an ae of 0.7 . These step spectra have been scaled up by a factor of 10 .
?[ { -/ +/' AEspectrum */: ( Spectra 0 ; pi * 1e-7 * Planckl[ WL ] x ) } ; 0.0 ; 300 ]
255.74
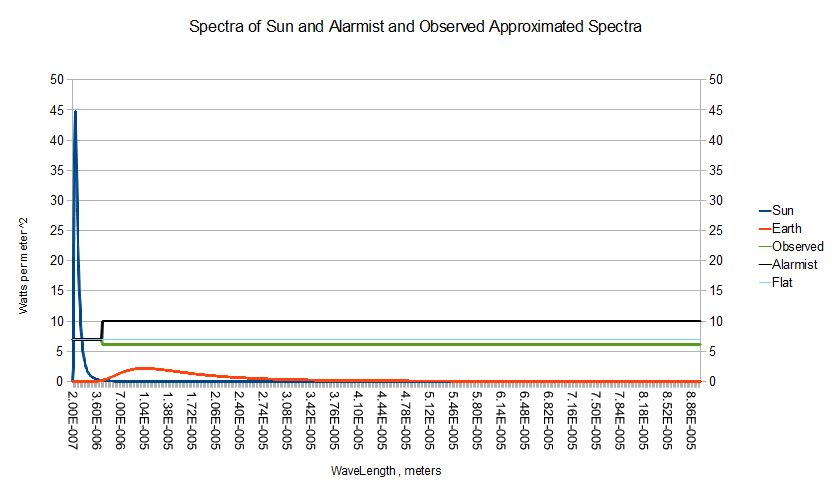
The crudeness , extremity , and hand waving justification of the Alarmist step function hypothetical surface spectrum strikes me as simply retarded versus any other branch of applied physics . It continues to be parroted year after year . Surely a better estimate of earth's mean surface spectrum is available ? Give me any spectrum , and I'll give you it's equilibrium temperature . The spectrum of liquid water would be a good start since it dominates the surface of the earth . The spectrum , and therefore equilibrium temperature of snow would be another interesting one . Would "snowball earth" be stable ? It's significant that the gray temperature is about 4 degrees warmer than the one obvious tipping point around , the freezing point of water .
With these sorts of computations , the delta in temperature from a delta in atmospheric CO2 should be straight forward to calculate .
But the earth surface is far from a uniform color . Nor are clouds uniformly distributed . But it takes little more code to add a Lambertian cosine function and individual spectra to any partitioning of the sphere .
I hope the computations here make it believable that a rather detailed planetary model could be expressed in not more than a couple of pages of succinct , and therefore transparent , definitions which bright high school students as well as researchers could explore for themselves .


 |
 |
||||
