|
BAA Vector Article Example of APL as a Tool of Thought |: Computation that for Venus's surface temperature to be due totally to the energy it receives from the Sun it would have to be 10 times as reflective in the IR as aluminum foil . “Any field of study that has the word science in it probably isn’t.” Ken Iverson , from Arthur Whitney , Memories of Ken APL is a disruptive technology . It sure has disrupted my life ; it and a smashed leg in a rock hubris error . And it is yet again . To cut to the chase , It's one thing to assert something ; its another to compute it .
The absurdity that our restoring to the biosphere some of the CO2 upon which it is built from previous extremely lush epochs will cause our global temperature to rise catastrophically , a " runaway greenhouse effect " , embraced by governments around the world , continues to do harm to both human and planetary welfare . Al Gore's witch doctor James Hansen unleashes as the ultimate threat Venus's extreme surface temperature . I recently implemented a vocabulary of the most basic physics needed to Calculate the Temperature of a Radiantly Heated Colored Ball . When a question arose in a blog about Venusian Mysteries , having the computations at my fingertips , I applied them to Venus . Here are the computations . They are rather simple . They are in Arthur Whitney's K 2.91 2002-03-22 , honed in my CoSy interactive computing notebook environment . The most basic computations of the temperature of a gray ball in our orbit are at Heartland.org's ClimateWiki Category:Essential Physics . That page has tables waiting for translations into more accessible languages . The computations : There are three relationships , other than geometry , which come into the computation of the equilibrium temperature of an externally heated opaque colored ball :
Computations in energy are linear . The energy density at a point in space can be calculated by simply summing the energy impinging on it from every direction around it . In our orbit , that total energy , essentially all from the sun , adds up to about 342. watts per square meter corresponding to a temperature of about P2Tsb 342 />/ 278.7 / kelvin . />/Our commonly asserted observed mean temperature is about 288.8 , about 3% warmer than this gray body temperature , up from about 288 ( 0.3% ) since before the industrial revolution . Actually , there seems to be about a degree uncertainty in that base temperature with the value 287. being put forward also . 1. Starting at least with experiments by Ritchie in the 1830s , and codified by 1860 by Gustav Kirchhoff and similarly by the lesser known Balfour Stewart , at any particular wavelength , a good absorber is a good emitter . In modern terms , the tendency for radiant energy of a particular wavelength to jiggle an atom or molecule or whatever transducing radiant to thermal energy is the same as the tendency for the atom , whatever , jiggling with a particular thermal energy to radiate at that wavelength . They are just two directions thru a symmetric filter . I'll call this parameter ae The color of an object is determined by it value of ae across the electromagnetic spectrum . An object looks blue if it reflects , does not absorb , blue more than other wavelengths . 2. The crowning achievement of 19th century physics was the derivation of the Planck function , the radiant spectrum of an object with ae = 1 across the entire spectrum as a function of its temperature . The inability to explain the function without assuming discrete oscillators , along with Einstein's related explanation of the energies of photoelectric emitted electrons , ushered in the quantum age . The table below presents the Planck function in terms of wavelength along with the utility function apv to create lists of wavelengths .
We will start by calculating the energy spectrum for the sun in our orbit , and that of a body whose temperature is such as to emit the same total energy . First we create a list of wavelengths in the range of interest . WL : apv[ .1 ; .1 ; 900 ] * 1e-6 / WaveLengths ; m % cycleCreates a list of 900 wavelengths , WL , from 0.1 microns to 89.9 microns is steps 0.1 micron . We will use the commonly cited temperature of 5778 kelvin for the sun's temperature , and the temperature of a gray ball in our orbit , 278.68 , calculated to match the same total energy using the StefanBoltzmann law as we'll see in a moment . Spectra : pi * 1e-7 * Planckl[ WL ]' 5778 278.68This expression produces a 2 column table of power density for each of the wavelengths in WL for each of the 2 temperatures . The 1e-7 scales the power to the 0.1 micron increment . Note that Planckl returns power per steradian while the total energy is emitted over a hemisphere . Thus the factor of pi . We can test the accuracy of these curves and the extent to which our range of wavelengths is sufficient to include all the energy by comparing the sum across the Spectra to the total energy calculated by Stefan Boltzmann . ( +/' Spectra ; T2Psb 5778 278.68 )
%/ r />/ 0.9999106 0.9922434 / in K.CoSy r holds the last result .You can see above what an enormous difference in radiance that 4th power StefanBoltzmann relationship creates . In fact , the sun radiates more than 500 times as much as a 279k ball even at the ball's peak wavelength around 10 microns . But the disk of the sun covers only 5.41e-6 of the total celestial sphere . Scaling the sun's spectrum by that factor , Spectra : Spectra * 5.41e-6 1.0 we get the blue and red curves below . 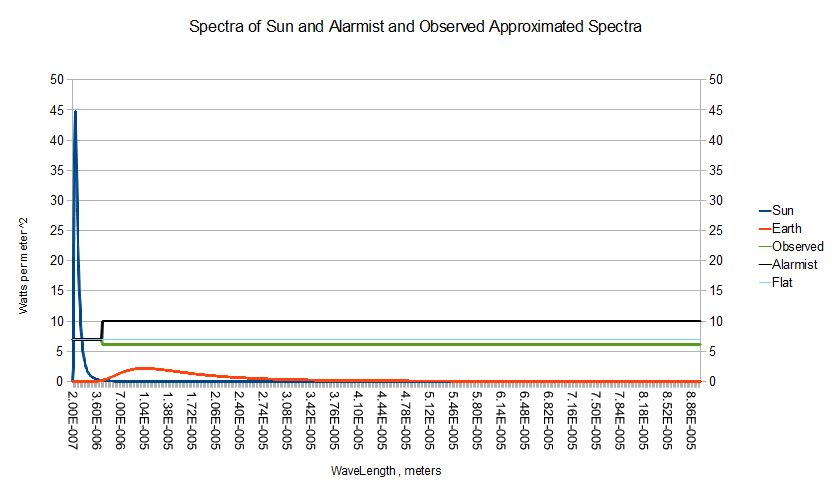 Their areas match . Thus the total energy coming from the sun in our orbit equals the total energy emitted by a ~ 279 black ball . Colored Balls Given the ae spectrum for any colored ball , its equilibrium temperature is that of a ball whose temperature is such that the inner product of its spectrum with Planck's thermal radiation spectrum equals its inner product with the sun's . That is , 1 ~ =/ +/' ObjSpectrum */: ( Sun ; Planck *' ( Sun ( Sun ?[ { -/ +/' ObjSpectrum */: ( SunSpectrum ; pi * Planckl[ wl ] x ) } ; 0.0 ; 300 ] The commonly asserted value for the lumped earth+atmosphere absorptivity with respect to the sun's spectrum is 0.7 . I have plotted three spectra across the graph above scaled up by a factor of 10 . All have a value of 0.7 over portion of the spectrum where the Sun dominates . The area The temperature For a gray , ie , just leaving that variable out of the equation . Let me indulge in a bit of history ; APL cost me my PhD . Getting Climate Science back on the path of experimentally testable quantitative CursorHoverComment |
|||||||||||||||||||||||||||||||