The
question I ask myself is, "What can I put in an oven to make the oven
hotter?"
The question I started with was "Will a black ball
stay warmer than a white ball in the freezer ."
To theoreticians this may seem a childish sort
of inquiry, but to empirical me it encapsulates
the whole matter of "radiative forcing." The initial condition is that
of hot coils warming the air inside (principally nitrogen) by
convective/conductive transfer (trace gases like CO2
included) and possibly by a small amount of radiative transfer to
IR-sensitive gases
that have not been otherwise excited. This is an obvious analog of the
earth's
surface heating the atmosphere.
Now,
the paradigm has it
that adding IR-sensitive gases like water vapor or CO2 will enhance the
radiative transfer of heat
from the coils (surface) to the chamber (atmosphere) and that this
effect
reverberates, making not only the chamber warmer but the coils too. For
the
life of me, though, I can't imagine how.
As greenhouse operators know, for instance,
water vapor REDUCES an enclosure's temperature.
It's a simple matter of water molecules storing heat in latent form and
making
less sensible heat available to the surroundings. Indeed, greenhouse
operators find that the benefit of mechanically removing WV from
the system outweighs the cost of trying to heat it. Dry air is just
easier to
keep warm. So discount the water molecule as a heat promoter.
CO2 then? Well, as an
IR-absorber it's pretty
weak, intercepting maybe 8% of the radiant energy the oven is
generating. Thus,
if infrared absorption and emission is the mechanism that raises the
chamber's
temperature, wouldn't a full-spectrum turkey do better?
But
you could roast that
turkey till it's a charred cinder, making it a virtual blackbody, an
IR-radiator to the nth degree... nothing will change. The turkey's
radiation is
just a response to what the oven coils are providing, and the oven's
temperature stays basically the same, if now a little lower due to the
extra
mass being heated.
To
cut to the chase, the
originators of greenhouse theory confused radiative absorption with
"blockage." IR-opacity merely denotes responsiveness, and
responsiveness necessitates emission. Appearances to the contrary, an
absorption line in spectroscopy doesn't signify "trapping," only
radiative
dispersal.
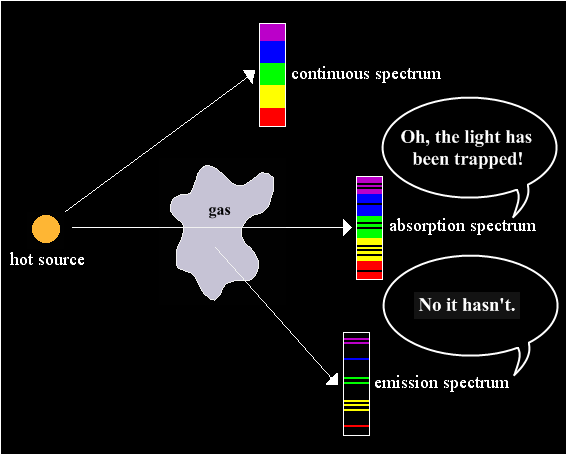
What's
absorbed isn't
TRANSMITTED (uninterrupted light passage) but is instead EMITTED
(transformed
to multiple paths). No energy is lost.
Certainly,
as a heated air
mass the atmosphere radiates, and this radiation can be observed from
the
ground below. But nothing in thermodynamics permits this so-called
back-radiation to heat the very object that's heating it. The oven
heats the
turkey; the turkey doesn't heat the oven. In other words, the second
law
prevails. Of course when you turn the oven off (nighttime falls) the
mass
that's been heated will help sustain the chamber's temperature. But
this
pertains to the chamber's contents per se (the atmosphere at large)
not to any
special property of trace gases. The energy spent in heating a mass by
day is
partly paid back at night, that's all. But a net GAIN of thermal
energy can't
be found anywhere in this picture. In other words, then, the first law
prevails.
By
conceptual default, I
bought into greenhouse theory when I started to research global
warming. I soon
learned from AGW skeptics, after all, that the dispute wasn't whether
more CO2 would lead to
warming but only by how much.
So when Gerlich and Tscheuschner came along, I merely considered their
ideas
interesting. But as my research progressed I began to notice that the
"negative feedbacks" proposed by AGW skeptics were becoming as
outlandish as the "tipping points" of alarmists. Where was any
solid evidence for either kind of effect? Was the theory under debate
even
valid?
The
answer is no. By
reducing convective heat loss the originators of greenhouse theory
observed
higher temperatures inside their glass boxes. But they THOUGHT that
they were
reducing radiative heat loss because glass is a selective absorber,
like CO2. Thus did a
human misperception become a
thundering law of nature: Restricting radiation induces a higher
temperature -
period. There are no brakes on this theory, however, no limits. And no
formula
to define it in any physics textbook. "With a radiative input of 1000
watts per square meter and an output of 50%, what temperature will a
blackbody
reach?" There SHOULD be an answer to such a basic question. Yet there
isn't. The formula is missing from physics textbooks because the
phenomenon of
radiative forcing does not exist. .